Using LED as a light source, researchers at Osaka University have now developed a convenient light-driven process for oxidizing polypropylene (PP) without harmful waste.
The research team used radicals to make the plastic react. Scholars chose an LED lamp as the light source photo-chemically activate radicals for breaking down the chemical bonds which make plastic unreactive and hard to decompose.
"In applications like printing and medical materials, plastics must be surface-modified," explained Tsuyoshi Inoue, co-author of the study. "Oxidizing C-H bonds is a textbook case in organic chemistry. With polymers, however, the risk is that anything strong enough to do this may also break the C-C bonds of the main chain, ripping the polymer apart. Luckily, the ClO2* radical is selective to react the side chain."
As a result, while the bulk polymer remains intact, the surface now bears a multitude of carboxylic acid groups (-CO2H), with major effects on the chemical reactivity. For example, the colorless plastic can now be stained with cationic dyes, such as Rhodamine B or Brilliant Green, which react with the anionic carboxylate ions. The originally water-repellent surface also becomes more hydrophilic.
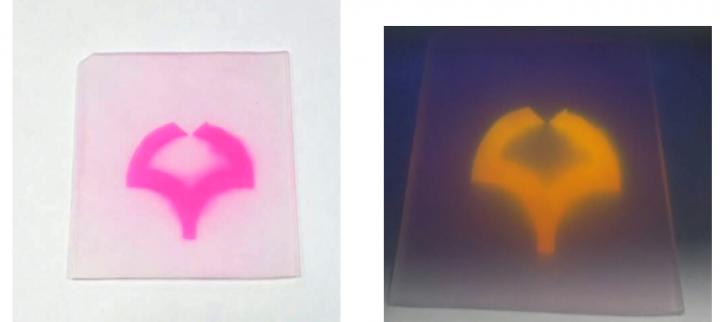
(Spot staining after treatment with rhodamine as a red ink after site-selective photooxygenation. Spot emission under black-light irradiation.)
"The reaction actually proved to be doubly selective for our purposes," said leading author Kei Ohkubo. "Not only did it cleave the C-H instead of C-C bonds, it specifically oxidized those on the side chain, even though they are stronger than those on the main chain. This is because the oxidation step, involving O2, is most favorable when the target for oxidation is CH2* ."
Previous methods for oxidizing olefinic polymers such as PP and polyethylene were either poorly controlled or highly polluting. The new process is thus the first clean and convenient solution to this problem, and may prove to be a valuable industrial tool in the customization of synthetic plastics.
The article, “Photochemical C–H oxygenation of side-chain methyl groups in polypropylene with chlorine dioxide,” was published in ChemComm.












