Scientists from MIPT’s Laboratory of the Biophysics of Excitable Systems have discovered how to control the behavior of heart muscle cells (cardiomyocytes) using laser radiation; this study will help scientists to better understand the mechanisms of the heart and could ultimately provide a method of treating arrhythmia. The paper has been published in the journal PLOS ONE.
“Right now this result may be very useful for clinical studies of the mechanisms of the heart, and in the future we could potentially stop attacks of arrhythmia in patients at the touch of a button,” says the corresponding author of the study and head of MIPT’s Laboratory of the Biophysics of Excitable Systems, Prof. Konstantin Agladze.
The work started with Japanese colleagues in Kyoto, but end results have been accomplished only now in MIPT. Prof. Agladze and his colleagues from the laboratory are researching cardiac engineering. In particular, his team succeeded in growing heart muscle tissue on a substrate of “spider silk”. The scientists have now moved from growing muscle tissue to finding ways of controlling it.
 |
|
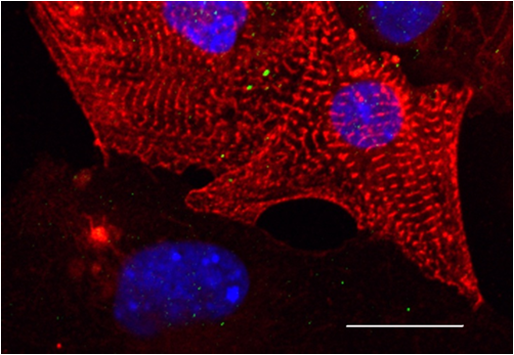
Fig.1 Micrograph of heart muscle cells (cardiomyocytes) (Photo courtesy of MIPT) |
Functional disorders in the heart muscles, particularly arrhythmia (an irregular heartbeat), are among the most common cardiac pathologies. One in eight deaths in the world is caused by acute arrhythmia. In order to study this type of heart disorder, it is important to be able to create “arrhythmia in vitro”, which is what azoTAB (azobenzene trimethylammonium bromide – a modified version of azobenzene) is used for.
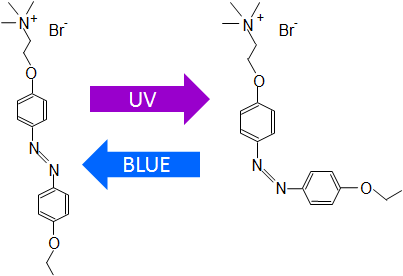
Its molecule consists of two benzene rings connected by a bridge of two nitrogen atoms. If the molecule is irradiated with UV light, the benzene rings change position relative to one another, they “fold”, and under the influence of visible light the rings return to their original configuration. An azoTAB molecule can therefore exist in two states, switching between them under the influence of radiation.
Prof. Agladze and his colleagues “taught” the azoTAB molecules to control cardiomyocytes so that one configuration did not prevent voluntary contractions (passive), and the other (active) “deactivated” contractions. Using a device similar to a projector, but with a laser instead of a lamp, the scientists created at each point the required concentration of the active form of azoTAB. This enabled them to control the cardiomyocytes in each specific point of the heart. However, the precise mechanism of action of azoTAB on the cells remained unclear.
The scientists have now been able to explain how the different forms of azoTAB affect cardiomyocytes.
 |
|
Fig.2 Diagram of the azoTAB isomerization. (Image courtesy authors of the study) |
Ion channels are used to transfer “commands” from one cell to another; they act as “gates” allowing ions to pass through cell membranes. In cardiomyocytes there are various types of channels capable of allowing potassium, sodium, or calcium ions to pass through. Agladze proposed that azoTAB affects the permeability of some of these channels. The scientists conducted an experiment on heart muscle cells that were placed in a solution of azoTAB in two different concentrations. They were then exposed to light of different wavelengths in the range of near-UV light. When each of the channels was examined, the two others were deactivated using inhibitor substances and the cardiomyocytes were isolated from one another.
It was found that after three minutes of exposure to the active form of azoTAB, the current through the calcium and sodium channels reduced by more than two times, and in the potassium channel it increased one and a half times. And after the azoTAB was removed by washing the cells, the function of the ion channels quickly returned to its normal state.
The experiment showed that the effect of azoTAB on a cell is reversible. This will mean that the results of the experiments will be able to be used in research and clinical practice, which could potentially lead to an effective treatment for arrhythmias.













