Scientist at University of California, Berkeley (UC Berkeley) have created a blue LED from a new semiconductor material halide perovskite, overcoming a major barrier to employing these cheap, easy-to-make materials in electronic devices.
Published in Science Advances on January 24, the study demonstrates the technology breakthrough of applying the unstable material as halide perovskite changes with temperature, humidity and the chemical environment and their optical and electronic properties are thus disrupted.
Making semiconductor diodes that emit blue light has always been a challenge, noted Peidong Yang, UC Berkeley chemist who led the research. So far, red- and green-emitting diodes made from perovskites have been demonstrated, but not blue. Halide perovskite blue-emitting diodes have been unstable, that is, their color shifts to longer, redder wavelengths with use.
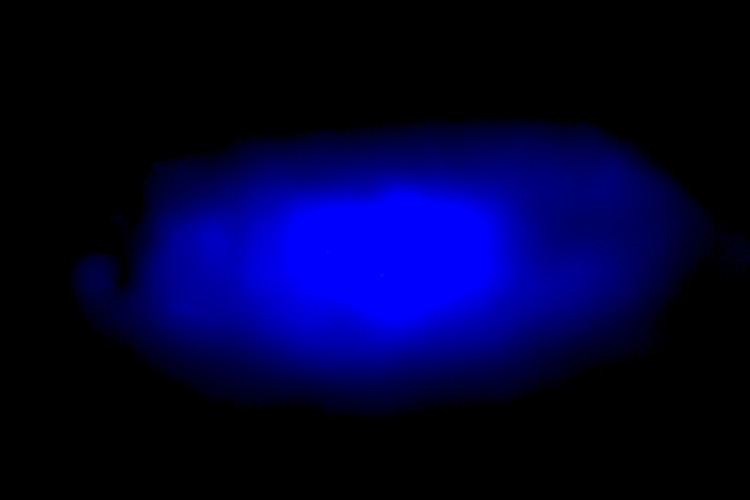
(Image: UC Berkeley)
The researchers found out that the instability of the material is due to the unique nature of perovskites' crystal structure composed of metal and halide atoms. When these elements are mixed together in solution and then dried, the atoms assemble into a crystal. Using a new technique and the ingredients cesium, lead and bromine, the UC Berkeley and Berkeley Lab chemists created perovskite crystals that emit blue light.
The team also discovered that light emitted by these crystals depends on the arrangement of and distances between atoms, the color changed with temperature. A perovskite crystal that emitted blue light (450 nanometers wavelength) at 300 Kelvin suddenly emitted blue-green light at 450 Kelvin.
The color changing feature of the blue-emitting perovskites with temperature can lead to interesting applications, said Yang. Two years ago, he demonstrated a window made of halide perovskite that becomes dark in the sun and transparent when the sun goes down and also produces photovoltaic energy.
"We need to think in different ways of using this class of semiconductor," he said. "We should not put halide perovskites into the same application environment as a traditional covalent semiconductor, like silicon. We need to realize that this class of material has intrinsic structural properties that make it ready to reconfigure. We should utilize that."





 CN
TW
EN
CN
TW
EN






