A joint research conducted by Japan’s Kobe University and Ushio, the Japan-based LED component maker, has provided proof for the first time in the world that direct and repetitive illumination from 222nm UVC radiation, which is a powerful sterilizer, does not cause skin cancer. This suggests that 222nm UVC is also safe for human eyes and skin. This technology is expected to have a wide range of antibacterial and antiviral applications in medical facilities and daily life.
These research results were published online in Photochemistry & Photobiology, titled “Long-term effects of 222nm ultraviolet radiation C sterilizing lamps on mice susceptible to ultraviolet radiation” on March 29 and will be presented at the ‘American Society of Photobiology 2020 meeting’ in Chicago on June 28.
UVC wavelength of 200-280nm is germicidal and has been widely used for disinfection. But UVC radiation is also harmful to human skin with its penetrative power. Researchers at Kobe University and Ushio, however, found that a smaller wavelength of 222nm is comparable to 254nm in terms of ability to eradicate bacteria on human skin and it does not cause skin cancer.
The team exposed mice to UV radiation in different groups, one group under a 222nm germicide lamp and the other to UVB (280-315nm). The mice exposed to UVB developed skin cancer and displayed adverse effects but mice in the 222nm germicide lamp group did not develop skin cancer at all. The effect on their eyes was also investigated and showed no abnormalities, even under a microscope.
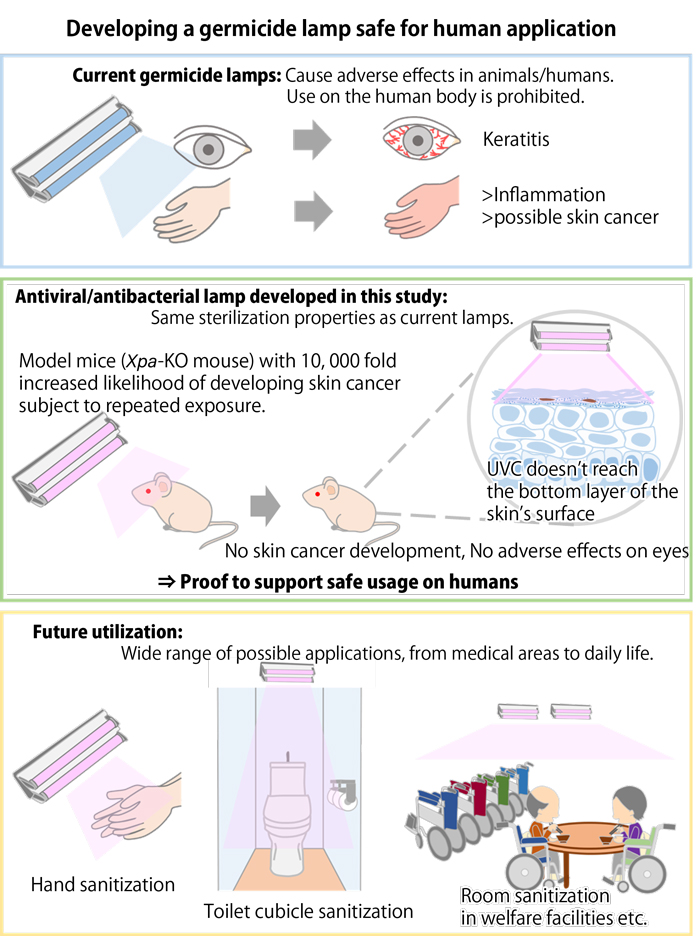
It is thus concluded that 222nm produced no adverse effects due to the level of skin penetration. 222nm UVC does not damage the DNA of skin cells because it only travels as far as the stratum corneum, the outermost layer of the skin.
Since UVC radiation with a wavelength of 222nm is powerful enough for disinfection and does no harm to human skin, it is expected that this technology may enable a wide range of antiviral and antibacterial applications.












